lv reversible vs unreversible | example of reversible and irreversible process lv reversible vs unreversible From our definitions of reversible and irreversible pressure–volume work, we . On 17 May, the French luxury label released the long-awaited ad for its Bleu de Chanel men’s fragrance. The 90-second spot – directed by Martin Scorsese – features the 28-year-old Oscar .
0 · reversible vs irreversible work
1 · reversible vs irreversible volume
2 · reversible vs irreversible process
3 · reversible vs irreversible pressure
4 · reversible vs irreversible gas
5 · reversible vs irreversible
6 · irreversible process vs reversible state
7 · example of reversible and irreversible process
6.9 / 10 97 Ratings. Acqua Fiorentina (2009) is a perfume by Creed for women and was released in 2009. The scent is fruity-fresh. The production was apparently discontinued. More. We may earn a commission when you buy from links on our site, including the eBay Partner Network and Amazon. Main accords. Fruity. Fresh. Floral. .
reversible vs irreversible work
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we . A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the .
We distinguish between two kinds of irreversible processes. A process that .
We distinguish between two kinds of irreversible processes. A process that .
A reversible process implies there is no entropy generated as the system moves between two .For reversible processes (the most efficient processes possible), the net change in entropy in .A reversible process is truly an ideal process that rarely happens. We can make certain .
In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection .
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the reverse path. An irreversible process is one in which the system and its environment cannot return together to exactly the states that they were in. We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process. We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.
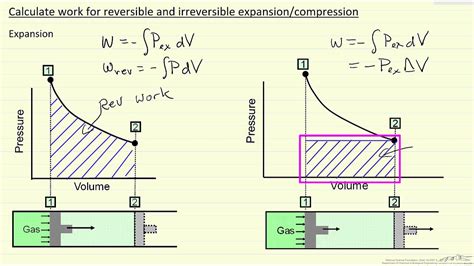
For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, . The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.
reversible vs irreversible volume
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection fraction (LVEF) by roughly 52%. 3 Similarly, a 36% drop in cardiac index and 34% increase in cardiac size have been shown to occur with rapid right ventricular pacing. 4.A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the reverse path. An irreversible process is one in which the system and its environment cannot return together to exactly the states that they were in.
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process. We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, .
The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.

Discover our great selection of Business Card Holders on Amazon.com. Over 68,000 Business Card Holders Great Selection & Price Free Shipping on Prime eligible orders
lv reversible vs unreversible|example of reversible and irreversible process